
Tesla Is Developing New Electrolyte Additives That Can Boost Electric Vehicles Li-Ion Batteries
Undoubtedly, the development of electric cars and artificial intelligence devices has changed our lives. These advances give hope to save the planet earth by reducing greenhouse gases emissions to zero. By this account, rechargeable batteries play a pivotal role in this area.
The role of lithium-ion cathodic materials such as nickel manganese cobalt (NMC) and nickel cobalt aluminum (NCA) is deniable in reaching today's position. When operated at an ordinary working potential of 4.2 V, these materials deliver excellent cyclic stability, but improving their discharge capacity is necessary for market requirements. Although discharge capacity can be enhanced by raising working potential, cyclic stability is lost.
Non-aqueous electrolyte solutions are usually used in current commercial Li-ion batteries. Lithium hexafluorophosphate (LiPF6) salt dissolves in organic carbonates solvents, in particular, binary or even ternary mixtures of ethylene carbonate (EC) with dimethyl carbonate (DMC), propylene carbonate (PC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC). The main drawback of these electrolytes is severe discharge capacity fade due to decomposition and transition metal ion dissolution on the cathode surface when cycling under a high working potential (beyond 4.2 V). Hence, introducing new electrolytes with high electrochemical stability under high working potentials is necessary for developing highly efficient, durable, safe, and low-cost batteries (for electric vehicles). These new electrolytes would be able to overcome carbonate-based liquid electrolytes’ problems. It seems that the solution provided by Tesla could probably pave the way in resolving this problem.
One of the world's most valuable companies and electric vehicle producers, Tesla is famous for its high-performance cathode materials such as NCA and NMC. The interesting point is that this company has been researching novel electrolyte additives such as difluorophosphates (DFPs), Dioxazolones, and nitrile sulfites compound for electrolytes in recent years. These researches can improve the lifetime of lithium batteries and the efficiency of electric vehicles. In the following, Tesla’s patents regarding this area will be reviewed.
A careful search indicates that Tesla has filed four patent families (18 patents in total) regarding DFPs electrolyte additives in Li-ion batteries since 2017. All these patents have been supervised by top inventor Mr. Jeff Dahn, the Dalhousie University and Novonix Company professor. These patents have been filed in the main markets of the battery industry such as Canada, the United States, the European Patent Office (EPO), China, Japan, and South Korea. One of the families was granted, two of them are in pending status, and the last one, which was only filed in the U.S., was rejected.
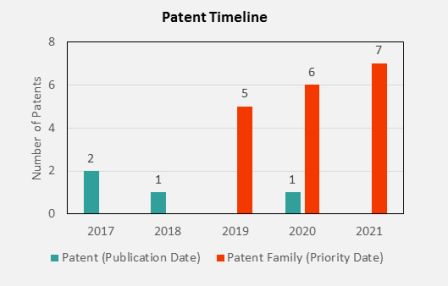
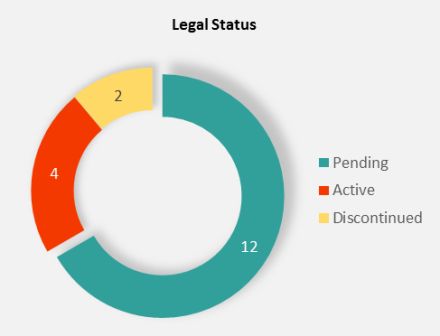
In these lithium difluorophosphate based patents, the electrolyte comprises a lithium salt with a non-aqueous solvent and an additive mixture (including lithium difluorophosphate and fluoroethylene carbonate or vinylene carbonate). One of them titled "Difluorophosphate additive compounds and methods thereof for use in energy storage devices," is specialized in electrolyte additives such as Lithium, Sodium, and Ammonium difluorophosphate. According to this patent, the Li-ion battery with difluorophosphates additives has an excellent remaining capacity after 2000 cycles at 40°C and a high voltage of 4.4V.
In summary, these patents tell us, the cyclability of a lithium-ion battery consisting of NMC cathode, graphite anode, and an electrolyte containing this additive has increased at high working potentials. But why? We will find the answer to this question using scientific resources.
The results of scientific researches indicate that by adding a small amount of lithium difluorophosphate to the organic electrolyte, a uniform protective layer modified by LiDFP can efficiently prevent electrolytes from decomposition on the NMC cathode surface. On the anode side, a stable and dense solid electrolyte interphase provided by LiDFP can protect the graphite anode surface exposed to electrolyte and efficiently suppress the decomposition of the electrolyte.
In the end, will the problems mentioned before be fully resolved with these Tesla’s patents? The answer depends heavily on cost and safety, but it is the beginning of big jumps.

