
U.S. Projects on Lithium-Sulfur Battery Technology
Increasing energy storage capacity is driving the demand for lithium-ion batteries. However, battery industry expansion and the growth of the electric vehicle market are hindered by the capacity limitations of specific chemistries, such as NCM cathodes, which contain nickel and cobalt.
To solve the issues, several technologies are candidates for replacing current chemistries. One of the most favorable types of batteries is lithium-sulfur batteries, which have a high specific energy thanks to their low-weight sulfur-based cathode. In addition, the lithium anode has a higher energy density than graphite anodes currently in use. In contrast to critical battery metals, sulfur is abundant, and there is no shortage or fluctuations in its price.
Several problems are associated with lithium-sulfur batteries, such as sulfur cathode volume expansion during lithium insertion. Since sulfur has a low electrical conductivity, it cannot trigger electrochemical reactions, so it has to be mixed with conductive materials, such as carbon to make electrochemical reactions possible. Sulfur cathodes also suffer from an irreversible reaction following the dissolution of sulfur species in electrolyte, decreasing available sulfur and lithium for charge/discharge and energy storage, decreasing battery cycling life.In the next decade, the U.S. Department of Energy will support five projects according to its roadmap focusing on Li-S batteries. Researchers at Stanford University and SLAC national laboratory have developed new cathode material based on lithium sulfide (Li2S) with a sulfide coating such as titanium sulfide (TiS2). The coating layer avoids sulfur dissolution in the solid-state polymer electrolyte composed of polyethylene oxide and lithium salt (PEO/TFSI).
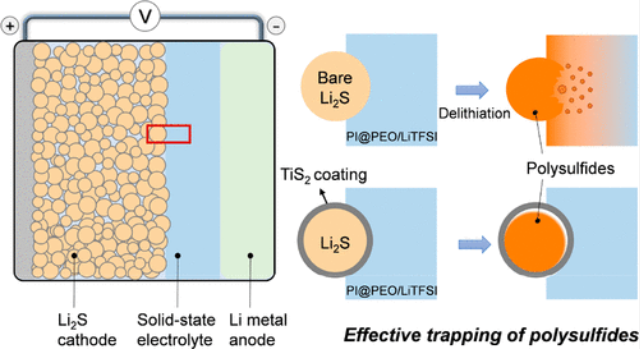
Nano Lett. 2020, 20, 7, 5496–5503
Because the activation energy barrier for sulfur cathodes is high, they require a high voltage to initiate lithiation. For example, this barrier was reduced by adding redox mediators (R.M.s) to electrolyte developed by Stanford researchers. Their purpose is to recover dissolved sulfur to the cathode so that it can be used to heal battery capacity when it is charged and discharged. Toward decreasing electrolyte waste, another project studies solutions to create sulfur electrodes with high energy density and low porosity. The active materials contain a conductive carbon network infiltrated with sulfur. Such a structure improves conductivity and electrode preparation. The other three projects deal with understanding the reaction between sulfur and the electrolyte and developing additives to control reactions between these two parts. There is currently more than $4.55 million in funding for lithium-sulfur projects, and they will continue to be funded until 2023.
An overview of 2022 projects of the U.S. Department of Energy was published by BattScout before.
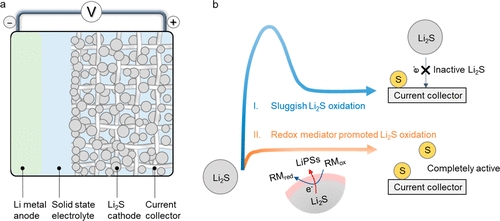
J. Am. Chem. Soc. 2021, 143, 43, 18188–18195

